|
Edwards Lifesciences Corporation (NYSE: EW), the global leader in the science of heart valves and hemodynamic monitoring, today announced it has received U.S. Food and Drug Administration (FDA) clearance for the ClearSight system.
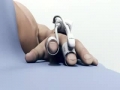
The ClearSight system is a noninvasive monitor that provides clinicians access to valuable blood volume and blood flow information for patients at moderate or high risk of post-surgical complications, in whom invasive monitoring would not be used.
To view the Multimedia News Release, go to http://www.multivu.com/players/English/7256451-edwards-fda-clearance-for-noninvasive-hemodynamic-monitoring-system/
Added:
3941 days ago by
MultiVuVideos
Runtime: 9m1s | Views: 964 |
Comments: 2
Not yet rated |
|